
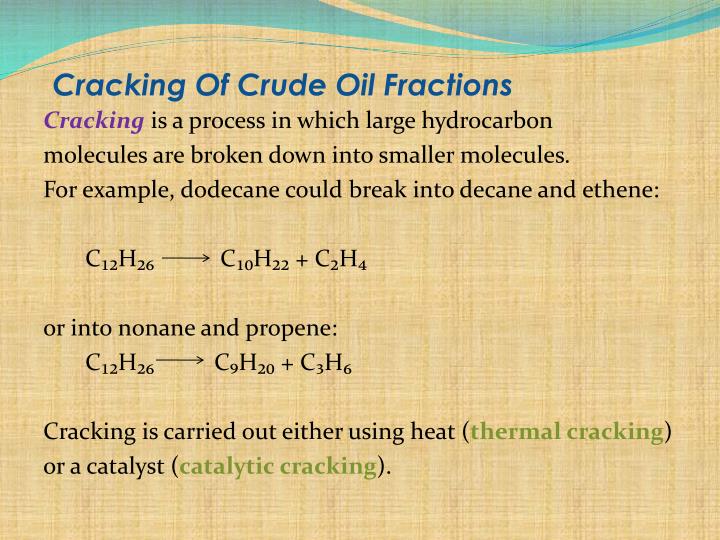
Cracking Of Decane To Form Ethene
Sony tv serial cid download in 3gp in mobile now download. Home » Top TV Serial Episodes » Top TV Serial Episodes - www.fullhd9.in. Tags: Watch and crime petrol download in 3gp mp4, Watch and adaalat episodes download in 3gp mp4, Watch and savdhaan india india fights back download in 3gp mp4, Watch and fear files download in 3gp mp4, Watch and star jalsha download in 3gp mp4, Watch and sony aath.
The large hydrocarbon molecules distilled from crude oil are difficult to vaporise and do not burn easily - so they. When we crack decane we produce ethene. The reaction of decane with heat and a catalyst to produce pentane, propene and ethene is an example of what? Thermal decomposition One of the products of cracking is pentane.
1) Find the longest chain of Carbon atoms in the molecule and base the name on the corresponding alkane. 2) Number the Carbon atoms in the longest chain - start from the end which will give the lowest numbers in the final naming. 3) Include the names of any substituent groups attached to the chain by name and the numbers of the Carbon atoms to which they are attached (alphabetical order if there is more than one.) 4) If the same alkyl group appears more than once as a substituent, indicate this by using 'Di-' 'Tri-' 'Tetra-'.
Doc Brown's GCE Chemistry Revising Advanced A Level Organic Chemistry A Level Revision Notes PART 10 Summary of organic reaction mechanisms A mechanistic introduction to organic chemistry and explanations of different types of organic reactions 10.2.3 The free radical thermal of alkanes What is the free radical mechanism for the cracking of alkanes? What free radicals are formed in cracking reactions of alkanes? What is the reaction mechanism for converting alkanes to alkenes? What is the importance of cracking to the petrochemical industry? How is cracking used in the oil industry?
The free radical mechanism for the cracking of alkanes to give lower alkanes, alkenes and hydrogen is given in a multi-step sequence and explained in detail. For GCSE/IGCSE/O level student notes see. Martin sigma mandolin f-style.
• What is cracking? Why is it done? • Cracking is a type of chemical reaction in which hydrocarbon molecules, usually saturated hydrocarbons called alkanes, are broken down into smaller molecules by the application of heat, pressure and catalysts. • Cracking is done to provide a greater variety of hydrocarbon molecules from oil fractions, that perhaps otherwise would be of little value.
Apart from the fact that oil does not contain valuable chemical stocks like alkenes, the distribution of alkanes in oil doesn't fit the commercial profile of hydrocarbon needs e.g. Not enough lower alkane petrol or diesel fuel molecules. • Typical example of a cracking reaction. • octane ==> hexane + ethene • C 8H 18 C 6H 14 + C 2H 4 • • Cracking reactions involve complex steps mainly involving the formation and reaction of free radicals - h9ighly reactive species with an unpaired electron. • What is the reaction mechanism of hydrocarbon alkanes being cracked to form alkenes, smaller alkanes and hydrogen? CH 3CH 2CH 3 ==> CH 4, CH 2=CH 2, CH 3CH 3, CH 2=CHCH 3, H 2 [mechanism ] • The equation is not meant to be balanced, but just to show the variety of possible products.
• Balanced equations e.g. Two possible cracking reaction outcomes of propane, and all will happen, but adjustment of temperature, pressure or catalyst can bias the product composition towards a desired outcome. • propane ===> methane + ethene • CH 3CH 2CH 3===> CH 4 + CH 2=CH 2 • propane ==> propene + hydrogen • CH 3CH 2CH 3===> CH 2=CHCH 3 + H 2 • See mechanism 31 below to illustrate the idea. • When alkane hydrocarbons are heated to a high temperature (450-900 oC, with/without superheated steam) they are thermally decomposed or 'cracked' to form mainly alkanes of lower C number, alkenes of equal or smaller C number and hydrogen. Mechanism 31 - free radical thermal cracking of alkanes • Cracking occurs via free radical reaction mechanisms and the diagram above illustrates the free radical reactions that can result from heating even a simple molecule like propane to a high temperature. • The free radical mechanistic steps show how alkenes, lower alkanes and hydrogen can all be formed.